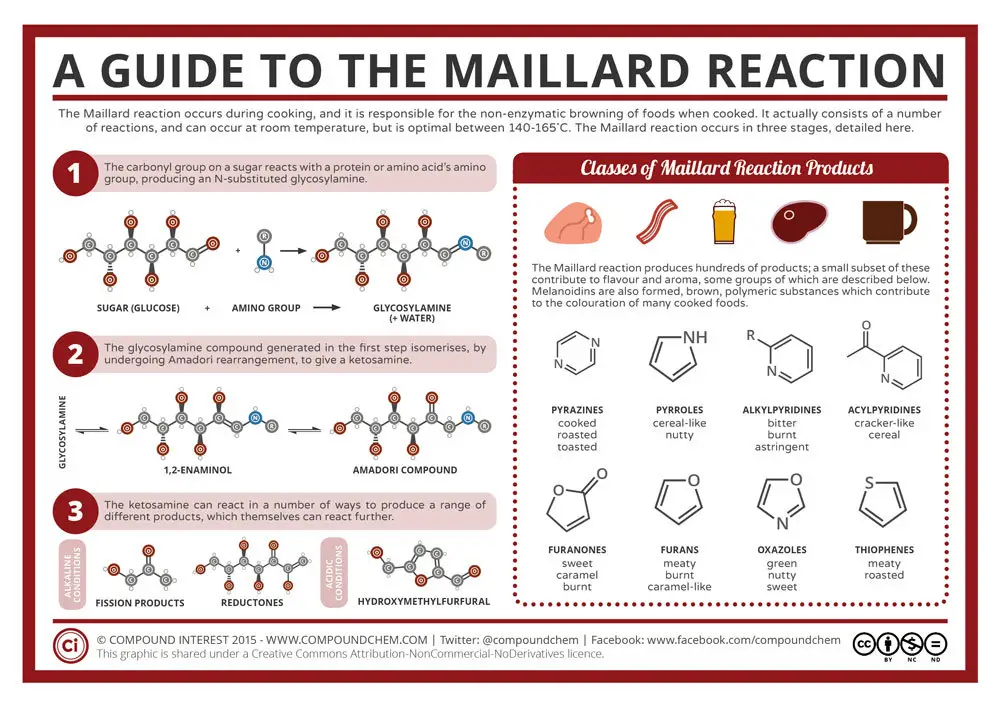
What is the Maillard Reaction: Why it Matters and Why Should You Care
Believe it or not, but chemistry plays an important part in cooking. In fact, cooking is a form of chemistry. This is due to the thermal energy released from the fire changing the composition of your food. One of the most important reactions out there, or at least important for us who are grillers, is the Maillard reaction.
In this article, we'll go over the Maillard reaction and go in-depth into the chemistry behind your foods!
More...
What are the Four Types of Chemical Reactions?
Before going in-depth into the Maillard reaction, let's talk briefly about chemical reactions! In short, a chemical reaction occurs when a compound is either made or broken due to interacting with another element, compound, chemical, etc. There are four types of chemical reactions, which we'll need to look over to better understand the Maillard reaction.
The four are,
What is the Maillard Reaction?
Now that we've gone over a brief chemistry lesson, and don't worry there'll be more chemistry in our article, let's look over the Maillard reaction. First off, how do you even pronounce it? Well, it's not pronounced how it's spelled; it's not "mail-lard", it's "my-Yard". The name comes from French chemist Louis-Camille Maillard, who gave the chemical reaction its name in the early 20th century.
The Maillard reaction is in fact a very ancient chemical reaction. As described by the Science of Cooking, the Maillard reaction is "a chemical reaction between an amino acid and reducing sugar, usually requiring the addition of heat".
Which is why, despite being credited, Louis-Camille Maillard didn't discover the process, only put a name to it.
Now, what type of reaction is this? It's grouped as a synthesis reaction, and we'll explain more in-depth with how this reaction works.
How Does the Maillard Reaction Work Exactly?
As a synthesis reaction, the Maillard reaction is a non-enzymatic browning, where the reactive carbonyl group of sugars interact with the nucleophilic amino group. In more layman terms, the Maillard reaction is what gives browned foods its distinctive flavoring, smell, and of course texture. What promotes the Maillard reaction you might ask? Well, it's due to heat.
Heat is a thermal energy, as we know, and when it comes into contact with food the resulting "cooking" is a chemical reaction. For the Maillard reaction, in particular, the heat forces different chemicals to bond and break apart, thus creaking new compounds. The heat acts as a catalysis for the reaction, and without the high heat, you wouldn't get the reaction.
Heat is important as it increases the Maillard reaction, while it will also decreases the reaction. The natural temperature that the Maillard reaction occurs is 140 to 165 °C or 280 to 330 °F. Most recipes, however, call for around 155 °C or 310 °F minimum, and if the temperature is too high then the food won't exactly brown as it will catch fire and burn.
Another factor playing a part in the Maillard reaction is the size of the food being cooked. For an example, suppose you're grilling some burgers and the grill's temperature is around 450 0F. Depending on the size of the burgers, and we'll say they're normal-sized, it will take around eight minutes or so for the burgers to be properly cooked. A larger slab of steak, on the other hand, will take around ten minutes or longer.
Taking into account the surface area of the meat if you need to make precise estimates and temperatures adjustments. Remember that a higher temperature will cook your food faster, but will burn it just as fast.

Why is the Maillard Reaction Important for Food?
The Maillard Reaction is important for food due in part to two reasons. The first reason is that is "cleans" the food, the second is that it produces new and unique flavors.
Cleaning:
By cleaning we just mean cooking. The Maillard reaction, just like every form of cooking, removes potential hazards from the food. Meat in particular easily becomes a hotbed for microbes, as the rich proteins and fats are ripe for the taking. The Maillard reaction heats the meat up to a high enough degree that most, if not all, of these microbes die.
Since washing meat isn't exactly useful, cooking the meat is usually the best way to remove anything that could harm you.
Flavor:
Next, we have flavor, which is arguably the most important thing that the Maillard reaction provides. Remember what we said about synthesis? The Maillard reaction is classified as a synthesis reaction, by creating newer compounds. Alternatively, compounds can be broken down into chemicals that provide a different flavor.
This is done by thermal energy, which breaks down existing compounds along with forming newer compounds by forcing chemicals to interact. Flavor scientist, and yes that is a profession, are very interested in Maillard's reaction. The flavors synthesized from the chemical reactions are used in artificial flavoring, and of course, chefs keep track of what flavors they can give their dishes with the right heat.
The Maillard reaction also plays an important part in the dairy industry as well, as the pasteurization process uses this chemical reaction to keep milk fresh and clean of microbes. This in turns gives the milk a much longer lifespan, or at least enough time to get to the supermarket and being put into a cooler. So yes, the Maillard reaction does work with milk. But what other foods can be affected?
Does the Maillard Reaction Affect All Foods?
Meat and milk, two foods that are affected by the Maillard reaction; what other foods might be affected? Well, turns out just about any food can and will be affected; as long as the food does meet the qualifications. This means the food needs to be dry to a degree. The wetter the food, the longer it'll take the Maillard reaction to go into effect; if it'll go to effect at all.
Sugar content also plays a key role, as the higher sugar content will lead to the caramelization process which we'll discuss more on below.
Caramelization and The Maillard Reaction
If you know your baking and sweet making, you likely heard about or maybe even worked with caramelization. It and the Maillard reaction do sound very similar and have a lot in common, but aren't the same thing. Looking at The Science of Cooking, caramelization "is the oxidation of sugar, a process used extensively in cooking for the resulting nutty flavor and brown color"
Still sounds similar, doesn't it? Let's look at it from a chemical angle. Caramelization is a decomposition reaction brought on by heat whereas the Maillard reaction is a synthesis which is also brought on by heat. In the chemical science field, decomposition isn't the same as food going rotten or bad; instead, it's when a more complex compound is broken down to simpler elements, as we mentioned.
That's the major difference between the two, outside of oxidation of course; caramelization breaks down compounds while the Maillard reaction makes new compounds or breaks down existing ones. Both use thermal energy, heat, to achieve this goal granted, but it's very hard to caramelize a steak compared to an apple. Although it's not out of the realms of impossibility, just very hard.
For caramelization to take place, you first need to take into account the sugar you're working with. For fructose sugar you'll need 110 °C or 230 °F. Galactoseis 160 °C or 320 °F, Glucose 160 °C or 320 °F, Sucrose 160 °C or 320 °F, and Maltose 180 °C or 360 °F. Whatever sugar you're using will greatly affect the temperature you need.
This is unlike the Maillard reaction in where, while sugar is present, a set temperature is usually good enough to get the reaction working. Now that we've covered caramelization, let's look at another important term when it comes to the Maillard reaction.
What Is This Non-Enzymatic Browning?
When it comes to browning foods, it can be achieved in two ways. Either enzymatic or non-enzymatic, or in layman terms an inside or outside source. The best example to look at is a banana. When exposed to the air, which is essentially all the time for a banana, it will gradually begin to ripen, going from green, to yellow, and then brown.
This is caused by the decay of the pigments that make a banana yellow, and said decay is brought on by a high concentration of ethylene. Because all of these enzymes and chemicals are present in the banana, this is an example of an enzymatic browning.
On the other hand, if you were to start cooking a banana on a grill or in a pan, it will begin to caramelize due to the high concentration of sugars present. The heat is causing the chemical reaction, which is why the browning is referred to as non-enzymatic. So, think of it as an inside and outside force causing the browning. Enzymatic is the inside force, and non-enzymatic is the outside source.
The Maillard reaction is an example of non-enzymatic reactions. The thermal energy is causing the changes, and not a breakdown of enzymes or compounds present in the food. Now, you might be curious if you can subvert non-enzymatic browning, or at the very least making the process slower. The answer is of course heat.
As long as you keep the heat low you won't have to worry about the non-enzymatic browning. Of course, there is an inherent risk in this as doing so might not cook your food properly. All said and done, non-enzymatic browning is just a simple chemical reaction in your food and it's perfect for trying out new flavors! Best of all, unlike enzymatic browning which actually makes your food go bad, non-enzymatic can be good for you!
Is the Maillard Reaction Dangerous?
While we say good for you, some might be wondering if the Maillard reaction or even browning food is bad for you? In recent years there has been a rise in studies and reports, by which we mean your friends posting on Facebook about a study they heard about from a source they don't tell you about, about the dangers of the browning food. Chiefly that it causes cancers and is overall unhealthy for you.
This is a little strange when you think about it given the Maillard reaction has been present in the human diet for thousands of years. Doing our part and actually researching the matter, we've found that the actual conclusion by the scientist, and not that one friend you haven't seen since grade school, is that the Maillard reaction is both good and bad.
The study we looked at concluded that the Maillard reaction provides benefits and negatives like,
So both good, and bad. The good is that besides your food tasting good, the process removes bacteria, fungus, viruses, and other possibly dangerous microorganisms from the food. The downside is that the process can release chemicals that are harmful to us, and possibly lower the nutritional value of the food. Such as is the case with vegetables and fruits.
So, the Maillard reaction is generally a case-by-case basis. For meat, it's a benefit because first off who would want to eat raw meat, and second off you get a much wider range of flavors and aromas. The downside is that a lot of meat in your diet is unhealthy for you, and the chemical compounds released by the Maillard reaction can cause medical side-effects such as cancer.
Before you panic, understand that you'd be needing to eat nothing but meat for decades along with not exercising to actually die of meat-related cancer. The Maillard reaction is safe, although you need to ensure your diet is in proper order and you get plenty of exercises to work off calories. The same goes for all foods that are browned. The biggest risk, the carcinogens, are very minimal and easy to off set.
The Maillard Reaction and You
We learned a great amount of the Maillard reaction, so how do we put this into effect? Well, it's quite easy! All you need is heat, and if you're using a grill there you go! You do need to take into account both the temperature of your grill, and the meats your planning to grill. Keeping your meats dried speeds up the Maillard reaction, and the cooking speed.
Wet meat produces steam when coming into contact with the grill, and steam rarely gets high enough in temperature to activate the Maillard reaction. Hence why smoking meat leaves it cooked, but at the same time still soft and tender; not characteristically crusty like you'd expect. Wet meat will also cause the local area to cool down, thus making the cooking time longer.
Braising meat will also affect the Maillard reaction. Since the meat is wet, it won't brown when cooking. To work around this, many chefs will sear slabs of meat before braising. Others, however, will actually attempt to cook their meat with the braise on as this can provide a much richer and complex flavor. What the Maillard reaction does, which many grillers love, is produce a crispy "shell" around the meats.
This shell keeps the juices and flavor on the inside, and normally keeping the meat when will provide this nice contrast. On the other hand, regardless if you're using rubs and braises that are higher in sugar or just keeping your meat wet with ice cubes, the cooking time will be longer compared to drier meats.
Another thing to keep in mind; the bigger the slab of meat, the longer it'll take to cook and get the proper Maillard reaction if that's what you aiming for. A slab of ribs will take longer to cook than a hamburger, after all. Sometimes this can work in your favor, such as with ribs; instead of keeping it right on the flame, keep it on the cooler end. The longer cooking time will ensure the entire rack is nice and crispy with a juicy inside.
And there you have the Maillard reaction in action! Or more specifically, chemistry in action. The Maillard reaction provides rich new flavors and a possible crispy exterior to trap those wonderful juices in! This leaves plenty of room for experimentation and to get new and exciting flavors. Best of all, the browning you see in the Maillard reaction also plays a part in our dairy, in our desserts, and even in simpler things like making toast.

All thanks in part to thermal energy reacting with the compounds present in your foods. Who knew that science played such a large role in the food you cook and grill?
Frequently Asked Questions (FAQ)
How Can you Use the Maillard Reaction to Your Advantage?
The Maillard reaction can be used to your advantage in a number of ways. Firstly, knowing how the Maillard reaction comes into play can help you keep your meats nice and juicy. Because the Maillard reaction comes into effect around 140 to 165 °C, or 280 to 330 °F, having your heat on lower or keeping your meats on a cooler side of the grill will reduce the crusting while keeping your food still tender.
Alternatively, you can start cooking wetter meats. Remember what we said about moist food and the Maillard reaction? Well, this can also work in your favor. The outside will become a crust, and the insides nice and juicy. The Maillard reaction will essentially create a shell around your food that keeps the moisture and juice on the inside; it's also handy for reducing juice spills too!
And for looser meats, like homemade patties, the Maillard reaction works perfectly to keep the content together and not falling apart on the grill.
How Can I Increase the Maillard Reaction?
With either heat or keeping your foods dry. For example, if you're making hamburgers and want them to be crispy, you'll want the temperature on the grill to be around 300 degrees Fahrenheit and your patties should have as little moisture as possible. The drier the food, and the hotter the flame, the quicker the Maillard reaction is to go into effect.
How Does Temperature Affect the Maillard Reaction?
The high heat produces more thermal energy which in turn breaks down existing compounds in the meat into simple chemicals which then start to fuse together. This in turn makes new and mouth-watering flavors. High heat will speed things up, but low heat will make the reaction take longer as you may expect.
Why Does the Maillard Reaction Give Food Odor?
Because compounds are breaking down and reforming thanks to the reaction, you have a plethora of smells occurring. Depending on the food being cooked, and its chemical nature, the resulting odors that are released can range from pleasant, to unnoticed, to smelling really bad.
Why Is the Maillard Reaction Important in Meats?
Two reasons. First, it cooks the meats; we don't exactly recommend eating raw meat, after all. Secondly, it produces that distinctive "crust" over the meats; like what you find in burgers and steaks. Is used right, you can have a shell around the meat thanks to the crust and the nice, tender, and juicy center.
What is the Chemical Equation for The Maillard Reaction?
The Maillard reaction isn't really a chemical reaction you can write don in a simple chemical equation. This is the full equation, which like said isn't very easy to write down. In essence, sugars are broken down and begin to fuse into new compounds.

Image source: https://www.compoundchem.com/2015/01/27/maillardreaction/
How Does the Maillard Reaction Affect Coffee During Roasting?
The same way it affects meat. It produces new aroma and flavor compounds the higher the temperature gets. Hence why coffee is "roasted"; that's not a fancy term, that's actually what happens! Sugars break down, react with amino acids in the coffee beans, and new flavors and aromas are born!
What Promotes the Maillard Reaction?
Heat and sugar. When the temperature gets just right, around 140 to 165 °C, or 280 to 330 °F, sugar compounds begin to break down; they're unable to remain stable in such high temperatures. As these compounds break down, they begin to merge with other compounds that were also broken. This is why the taste, texture, and smell are all so different before and after the Maillard reaction.
How Can You Stop the Maillard Reaction?
Typically, the best way to stop the Maillard reaction is to cook your food at lower temperatures. Seeing as this is a tedious task, or some foods require higher temperatures, you can easily make your food "wet" using either water or a braise. This will slow down the Maillard reaction while still allowing said food to be cooked.
How Can You Speed-Up the Maillard Reaction?
See above with "How Can I Increase The Maillard Reaction? ". High heat and dry foods will speed up the Maillard reaction.
What Is the Maillard Reaction in Milk?
In milk production the Maillard reaction is used for pasteurizing the milk; the high heat kills microbes, while also promoting the sugar and fats inside the milk to break down. It does not "brown" the milk like it would other foods, but keeps it clean long enough to be bottled and sold.
What Temperature Does the Maillard Reaction Occur At?
The Maillard reaction goes into effect around 140 to 165 °C or 280 to 330 °F.
Is the Maillard Reaction Unhealthy?
Yes and no. We discussed the issue in full, and while there is the risk of carcinogens when the Maillard reaction is at work the actual risk is extremely minimal. Not to mention this chemical reaction has been with us for thousands of years!
How Does pH Affect the Maillard Reaction?
To be fair, this one is too complex even for us. Still, it's our job to answer any questions you might have and so to give a very oversimplified explanation, the pH values tend to affect which types of compounds are formed and how the compounds react when the Maillard reaction is in effect. This includes carbon compounds, and how they play a part in the foundation of newer compounds.
To be fair, it's not something we'd worry about too much but still if you like your science and naming chemicals that tend to be unpronounceable, you'll enjoy reading up on it.
What Causes Maillard Browning?
Maillard browning and the Maillard reaction are the same things, and Maillard browning shouldn't be confused with enzymatic browning. Once again, the reaction is produced by heat breaking down sugars and then forcing them to build new compounds.
How Do You Slow Down Enzymatic Browning?
Enzymatic browning is caused by the natural breakdown of compounds in your foods. To keep this from happening, you need proper food storage or at the very least to remove as much moisture from the food as possible. Hence why drying, freeze-drying, and smoking are popular methods to protect foods from eventual decay.
Given that water plays a key part in enzymatic browning, the first thing to do is remove any and all moisture. The next thing to do is keep the food in a sterile environment where microbes won't interact with it. Even with all these methods in place, the chemical structure of your food will still begin to break down. Proper storage and preservation are to keep it lasting as long as possible before eventual decay.
Is Browning Food Bad for You?
Once again, yes and no. Browning produces carcinogens, but at such minimal levels, they're harmless.